Plant ß-1,3-Glucanases (PR-2)
Several classes of proteins, called pathogenesis-related (PR) proteins, are induced in response to the infection of plants with microbial pathogens. The family of PR-2 proteins, which are ß-1,3-glucanases (glucan endo-1,3-ß-glucosidases, E.C. 3.2.1.39) catalyze endo-type hydrolytic cleavage of the 1,3-ß-D-glucosidic linkages in ß-1,3-glucans. The ß-1,3-glucanases (ßGlu) are abundant, highly regulated enzymes widely distributed in seed-plant species.
Proteomic analysis of Arabidopsis showed that ß-1,3-glucanases are among the group of glycosylphosphatidylinositol-anchored membrane proteins (GPI-APs, Elortza et al. 2003). GPI-APs are proposed to be involved as enzymes and receptors in cell adhesion, cell separation and differentiation processes. This could be a new function of ß-1,3-glucanases during endosperm weakening.
In sea urchins ß-1,3-glucanases are reported as part of the embryo hatching enzyme (Bachman and McClay 1996).
Although the major interest in ß-1,3-glucanases stems from their possible role in the response of plants to microbial pathogens, there is strong evidence that these enzymes are also implicated in diverse physiological and developmental processes in the uninfected plant including cell division, microsporogenesis, pollen germination and tube growth, fertilization, embryogenesis, fruit ripening, seed germination, mobilization of storage reserves in the endosperm of cereal grains, bud dormancy, and responses to wounding, cold, ozone and UV B.
Direct evidence for developmental roles of ß-1,3-glucanases (ßGlu ) in plant reproductive biology were obtained by using transgenic approaches:
(1) Endosperm-ßGlu: Over-expression of ßGlu in the tobacco seed covering layers under the control of an ABA-inducible ßGlu-transgene provided direct evidence that ßGlu causally promotes endosperm rupture (review Leubner-Metzger 2003). In addition, ßGlu is involved in the the after-ripening-mediated promotion of testa rupture, a process that involves release of coat-enhanced dormancy (Leubner-Metzger 2005).
(2)
Anther-ßGlu: Transgenic plants demonstrate that ßGlu are causally involved in pollen development (references in review Leubner-Metzger and Meins, 1999)
(3) Style-ßGlu: Transgenic plants suggest that ßGlu are involved in fertilization (references in review Leubner-Metzger and Meins, 1999)
Functions and Regulation of Plant ß-1,3-Glucanases (PR-2) - Review
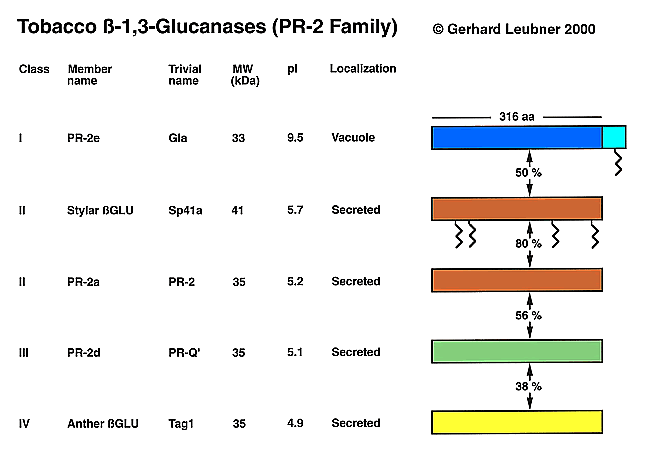
Structural Classes of ß-1,3-glucanases and PR-2 Nomenclature
Figure: Structural Classes of Tobacco ß-1,3-Glucanases
Table: PR-2 Family Members (ß-1,3-glucanases) of Nicotiana Species
ß-1,3-Glucanase causally contributes to endosperm rupture and after-ripening of tobacco seeds
ABA-sensitive ßGlu expression in the endosperm prior to endosperm rupture is widespread among Solaneceous seeds
|
Structural Classes of ß-1,3-Glucanases (ßGlu) and PR-2 Nomenclature
-
ßGlu exist as multiple structural isoforms that differ in size, isoelectric point, primary structure, cellular localization, and pattern of regulation. The most detailed sequence information for these isoforms is available from cDNA and genomic clones of tobacco ßGlu, which form a multigene family. Based on amino acid sequence identity, the various ßGlu of the genus Nicotiana have been be classified into four structural classes. The classification, nomenclature and salient features of these ßGlu are summarized in the Table and the Figure presented below. Review: Leubner-Metzger and Meins (1999) Functions and Regulation of Plant ß-1,3-Glucanases (PR-2)
-
The ca. 33 kDa class I enzymes (ßGlu I) of Nicotiana tabacum, which constitute the PR-2e subgroup of tobacco PR-2-proteins, are basic proteins localized in the cell vacuole. ßGlu I is produced as a preproprotein with a N-terminal hydrophobic signal peptide, which is co-translationally removed, and a C-terminal extension N-glycosylated at a single site. The proprotein is transported from the endoplasmic reticulum via the Golgi compartment to the vacuole where the C-terminal extension is removed to give the mature, ca. 33 kDa enzyme, which is not glycosylated. There is considerable indirect evidence that, in analogy to tobacco class I chitinases and barley lectin, the C-terminal extension contains a signal for targeting to the vacuole. Recent results obtained with cultured tobacco cells provide strong evidence that vacuolar class I ßGlu and chitinases can be secreted into the medium via a novel pathway.
-
The known mature ßGlu I of tobacco and Gn2 of Nicotiana plumbaginifolia share ca. 98 % amino acid identity. In contrast, with only ca. 76 % similarity, the Gn1 of N. plumbaginifolia is structurally more distinct. The tobacco ßGlu I multigene family consists of very similar homeologues derived from the N. sylvestris and N. tomentosiformis progenitors of tobacco as well as recombinants of the two progenitor types. The 35-kDa tomato ßGLU I isoform GluB shares ca. 90 % amino acid sequence identity with tobacco ßGLU I.
-
In contrast to ßGlu I, the class II and III members of the PR-2 family are secreted into the extracellular space. The tobacco class II ßGlu PR-2a, PR-2b, PR-2c and the class III ßGlu PR-2d, also known as PR-2, PR-N, PR-O, PR-Q', respectively, are acidic proteins without the C-terminal extension present in the class I enzymes ranging in apparent size from ca. 34 to 36 kDa in denaturing gels. The class II tobacco isoforms are at least 82 % identical in amino acid sequence and differ from the class I enzymes at a minimum of 48.8 % of the positions.
-
Class II also includes the two acidic 41 kDa stylar ßGlu isoforms, Sp41a and Sp41b, which are exclusively expressed in the style of tobacco flowers. They do not appear to be induced by pathogen infection and, hence, are referred to as "PR-like proteins".
-
The acidic ca. 35 kDa PR-2d (PR-Q') is the sole representative of tobacco class III ßGlu and differs in sequence by at least 43 % from the class I and class II enzymes. Two highly homologous cDNA clones for class III ßGlu have been isolated from tomato plants infected with a viroid. Based on deduced amino-acid sequences, TomPR-Q'a is an acidic isoform 86.7 % identical to tobacco PR-Q' and TomPR-Q'b is a basic isoform 78.7 % identical to tobacco PR-Q'.
-
Tag1 represents the class IV of tobacco ßGlu. It is a "PR-like" protein which is expressed specifically in tobacco anthers. Like the tobacco class I ßGlu, Tag1 is encoded by a small gene family with at least two members derived from the N. sylvestris and N. tomentosiformis progenitors of tobacco. Based on deduced amino acid sequence, Tag1 encodes a polypeptide with an N-terminal signal peptide, but no C-terminal extension, suggesting that the protein may be secreted. The mature form of Tag1 is an acidic, 35 kDa protein, which shares absolutely conserved sequences found in all classes of tobacco ßGlu. It is 37-38 % identical in sequence to the mature forms of tobacco class I Gla, class II PR-2, and class III PR-Q'.
|
|
Table 3.1: PR-2 Family Members (ß-1,3-Glucanases) of tobacco and Other Nicotiana Species
| Class (a) |
Member name |
Trivial name |
Origin (b) |
MW (kDa) (c) |
pI |
Localization |
| I |
PR-2e |
Glb |
Nt (T) |
33 |
Basic |
Vacuole |
| I |
PR-2e |
Gla |
Nt (S) |
33 |
Basic |
Vacuole |
| I |
PR-2e |
Gglb50 |
Nt (S) |
|
Basic |
Vacuole |
| I |
PR-2e |
Gln2 |
Nt (S) |
|
Basic |
Vacuole |
| I |
|
Gn2 (d) |
Np |
|
Basic |
Vacuole |
| I |
|
Gn1 (d) |
Np |
34 |
Basic |
Vacuole |
| I I |
PR-2a |
PR-2 (I, b4) |
Nt |
35 |
Acidic |
Secreted |
| I I |
PR-2b |
PR-N (b5), G19 |
Nt |
35 |
Acidic |
Secreted |
| I I |
PR-2c |
PR-O (b6(b), O') |
Nt |
35 |
Acidic |
Secreted |
| I I |
|
PR-2d |
Nt |
|
Acidic |
Secreted |
| I I |
Stylar ßGLU (e) |
Sp41a |
Nt |
41 |
Acidic |
Secreted |
| I I |
Stylar ßGLU (e) |
Sp41b |
Nt |
41 |
Acidic |
Secreted |
| I I I |
PR-2d |
PR-Q' |
Nt |
35 |
Acidic |
Secreted |
| I V (f) |
anther ßGLU (e) |
Tag1 |
Nt |
35 |
Acidic |
Secreted |
© Review "Functions and Regulation of Plant ß-1,3-Glucanases (PR-2)" by Leubner-Metzger and Meins (1999).
(a) Classification according to amino acid sequence identity of the mature proteins.
(b) Nicotiana tabacum (Nt); N. plumbaginifolia (Np); T and S refer to the N. tometosiformis and N. sylvestris progenitors of tobacco, respectively.
(c) Approximate molecular weight of mature protein; Selected values from the literature, which might differ between publications.
(d) Amino acid sequence identity to tobacco ßGLU I enzymes of the N. plumbaginifolia enzymes is ca. 98 % for Gn2, but only ca. 76 % for Gn1.
(e) Not induced by pathogens, i.e. a "PR-like protein".
(f) Tag1 is assigned to a new class, since it shares only 37-38 % amino sequence identity to Gla, PR-2, and PR-Q'.
|
| |
ß-1,3-Glucanase causally contributes to endosperm rupture and after-ripening of tobacco seeds |
|
- In tobacco class I ß-1,3-glucanase (ßGlu I) contributes to endosperm weakening and promotion of radicle protrusion, i.e. endosperm rupture. This can be either direct by degradation of ß-1,3-glucan (callose) cell wall material, e.g. at the neck regions of plasmodesmata or as a callose layer directly below the testa, or indirect by the release of elicitor-active oligosaccharides that induce cell-wall degradation, e.g. by the action of reactive oxygen species (ROS) that are known to cleave cell wall polysacchsrides and cause cell-wall weakening. Several aspects of the detailed molecular mechanism of ßGlu-action have been elucidated.
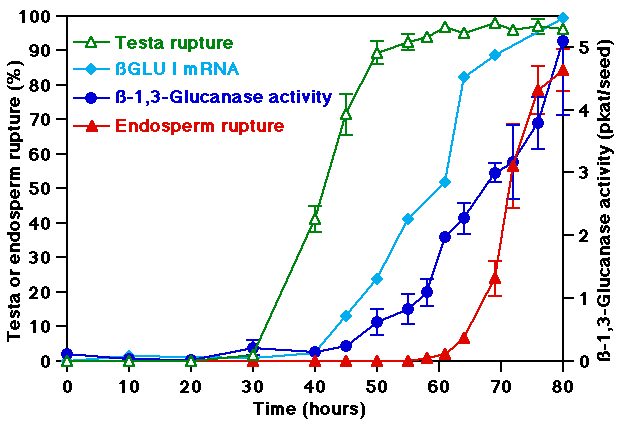
- ßGlu I is transcriptionally induced in germinating tobacco seeds just prior to endosperm rupture, but after testa rupture (Figure 1).
- This induction is highly localized in the micropylar endosperm at the site of radicle emergence (Figure 2).
- The induction of ßGlu I and endosperm rupture are tightly linked in response to physiological factors known to affect the incidence and timing of germination (Light, GA, ethylene, ABA).
- ABA treatment specifically delays endosperm rupture and inhibits the induction of the ßGlu I host genes (Figure 2). The onset of endosperm rupture depends on a critical threshold concentration of ßGlu I.
- Sense transformation with a chimeric ABA-inducible ßGlu I transgene resulted in overexpression of ßGlu I in tobacco seeds and promoted endosperm rupture of fresh mature seeds and of ABA-treated afterripened seeds (Leubner-Metzger and Meins, 2000; reviewed by Leubner-Metzger 2003).
- Sense- and antisense-ßGLU I-transformation of tobacco reveals a second target site of ßGLU-action that confers a maternal effect and affects testa rupture and after-ripening (Leubner-Metzger 2002, Leubner-Metzger 2005)
- Taken together these results provide direct evidence that ßGlu I contributes to endosperm rupture and seed after-ripening.
- ABA inhibition of ß-1,3-glucanase in the endosperm is widespread among Solanaceae seeds (Petruzelli et al. 2003).
- ABA inhibition of ß-1,3-glucanase is also evident in perisperm weakening of Cucurbitaceae seeds where a callose layer contributes to the semipermeability of the perisperm (Yim and Bradford 1998, Amritphale et al. 2005, Ramakrishna and Amritphale 2005).
|
 |
 |
|
Figure 1: The time course of ßGlu I induction during tobacco seed germination. ßGlu I mRNA, 33 kDa ßGlu I protein, ßGlu enzyme activity accumulate just prior to the onset of endosperm rupture, but after testa rupture. ßGlu I promoter activity in GUS reportergene seeds accumulates in the micropylar endosperm prior to its rupture. © Leubner-Metzger et al. (1995)
|
|
| |
|
|
 |
| |
|
|
|

|
 |



