Seed After-ripening: Dormancy release and promotion of germination
After-ripening, i.e. a period of usually several months of dry storage at room temperature of freshly harvested, mature seeds, is a common method used to release dormancy and to promote germination (Bewley, 1997; Finch-Savage and Leubner-Metzger, 2006; Kucera et al., 2005; Leubner-Metzger 2003). Seed after-ripening can be characterized by:
(1) A widening of the temperature range for germination.
(2) A decrease in ABA level and sensitivity and an increase in GA sensitivity or loss of GA requirement (see GA response figure below).
(3) A loss of light-requirement for germination in seeds that do not germinate in darkness (see GA response figure below).
(4) An increase in seed sensitivity to light in seeds that do not germinate even with light.
(5) A loss of the requirement for nitrate.
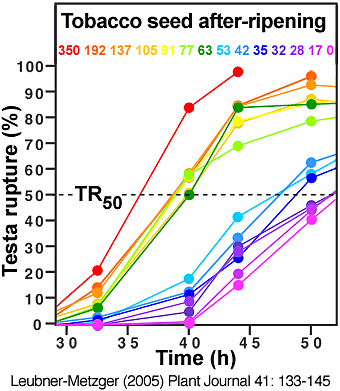
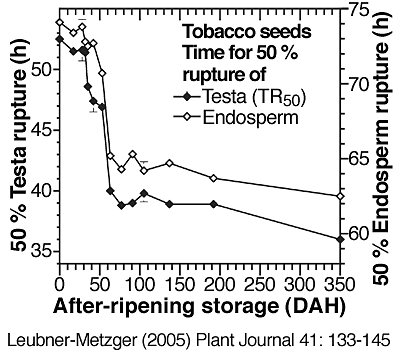
(6) An increase of germination velocity. During tobacco seed after-ripening this is evident by a promotion of testa rupture and endosperm rupture (see the two figures directly below).
|
 |
 |
 |
 |
 |
Time course analyses showing the promotion of of Nicotiana tabacum Havana-425 testa rupture and endosperm rupture during seed after-ripening, i.e. during prolonged dry storage at room temperature; DAH = days after harvest. (Leubner-Metzger, 2005)
Left: The time curves for testa rupture (TR) and the corresponding DAH numbers are indicated by rainbow colors. TR50 is the time needed to reach 50 % testa rupture.
Right: Times in h for 50 % testa rupture (TR50 from left figure) or endosperm rupture as determined from kinetic analyses of imbibed seed populations. Note that a decrease in these values represents a promotion of testa or endosperm rupture. The speed of tobacco testa rupture and endosperm rupture is equally promoted by after-ripening.
|
 |
The parameters that determine seed after-ripening are moisture and oil contents, seed covering structures, and temperature (Manz et al., 2005 and references therein). After-ripening is prevented in very dry seeds; it requires seed moisture contents above a threshold value. This threshold moisture content is species-specific and lower in oilseeds compared to starchy seeds because they contain less bound water when equilibrated at any given relative humidity. After-ripening is also prevented during storage at very high air humidity (higher equilibrium moisture content). For several species the conditions that generate optimal low-hydration values for after-ripening have been determined (Leubner-Metzger, 2005 and references therein).
The molecular mechanisms of after-ripening are not known. Non-enzymatic reactions that remove germination inhibitors, reactive oxygen species and antioxidants, membrane alterations, and specific protein degradation via the proteasome have been proposed (Finch-Savage and Leubner-Metzger, 2006 and references therein). Using cDNA-amplified fragment length polymorphism (cDNA-AFLP) gene expression analysis, Bove et al. (2005) provide evidence that Nicotiana seed after-ripening generates a developmental switch at the transcript level. This is in agreement with the A. thaliana Cvi transcriptome work (Cadman et al., 2006). In wild oat, transcriptional and post-transcriptional regulation are both important for the expression of dormancy-associated genes (Li & Foley, 1997).
Two recent publications provide evidence for gene expression in air-dry Nicotiana seeds during after-ripening. A rapid promotion of testa rupture of Nicotiana tabacum seeds occurs after ca. 60 days of dry storage (Leubner-Metzger, 2005). This was associated with transient ß-1,3-glucanase gene expression in the covering layer during tobacco after-ripening. Bove et al. (2005) found that at least eight specific mRNAs accumulated in air-dry, low-hydrated seeds of Nicotiana plumbaginifolia during after-ripening. Thus, while degradation of mRNAs and proteins for positive regulators of dormancy and for negative regulators of germination appears to be part of the molecular mechanisms of seed after-ripening, the possibility of de novo gene expression during seed after-ripening should also be considered.
|
 |
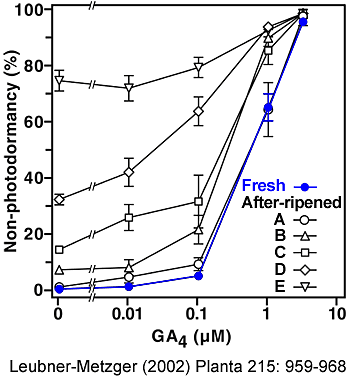
Gibberellin-requirement of photodormancy release of fresh and after-ripened Havana-425 tobacco seeds imbibed in darkness. (Leubner-Metzger 2002)
Fresh seed batches (blue circles) are completely photodormant in control medium, i.e. no germination occurs during dark-imbibition.
After-ripened seed batches (open symbols) were grouped into 5 equally-sized classes depending on their degree of non-photodormancy in control medium: class A 0-5 %, B 5-10 %, C 10-25 %, D 25-50 %, E 50-100 % germination during dark-imbibition, respectively. The percentage of germination of approximately 100 seeds was scored after two weeks of imbibition in darkness in medium without (control) or with 0.01, 0.1, 1, or 4 µM GA4 added. The GA4-concentrations for 50 % photodormancy release are 0.73 µM for fresh seeds, and 0.73, 0.2, 0.1, 0.02 µM GA4 for after-ripened seed batches of the classes A, B, C, D, respectively. |
 |
| |
|
|
 |
| |
|
|
 |
|
 |
| |
|
|
|

|
 |

