Plant Physiology 161: 1903-1917 (2013)
Spatiotemporal seed development analysis provides insight into primary dormancy induction and evolution of the Lepidium DELAY OF GERMINATION1 Genes [W][OA]
School of Biological Sciences, Plant Molecular Science and Centre for Systems and Synthetic Biology, Royal Holloway, University of London, Egham, Surrey TW20 0EX, United Kingdom (KG, AV, GLM);
Web: 'The Seed Biology Place' - www.seedbiology.eu
University of Freiburg, Faculty of Biology, Institute for Biology II, Botany/Plant Physiology, D-79104 Freiburg, Germany (KG, AV, ABM, GLM)
Universität Osnabrück, Fachbereich Biologie, Botanik, D-49069 Osnabrück, Germany (KS, KM)
Received December 24, 2012; Accepted Feburary 19, 2013; Published Feburary 20, 2013.
DOI:10.1104/pp.112.213298
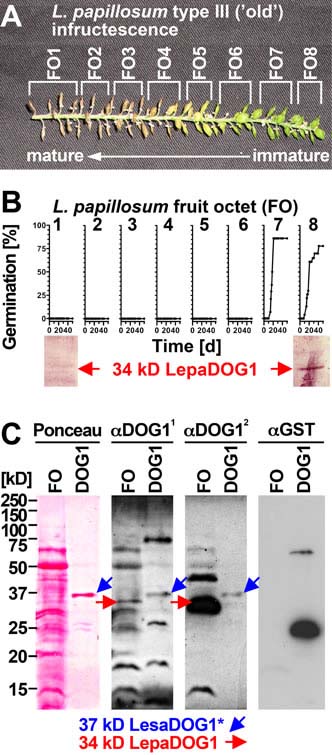 |
Figure 6. Analysis of LepaDOG1 protein expression within the Lepidium papillosum infructescence. A, Type III L. papillosum infructescence exhibiting the seed maturation gradient and the experimental dissection into fruit octets (FOs). B, Upper panel: FO-specific germination time courses to determine the developmental switch to seed dormancy. Lower panel: FO-specific immunoblot analysis of LepaDOG1 protein (arrow) expression using a polyclonal antibody (α-DOG11) raised against A. thaliana DOG1 (AtDOG1); C, Control experiments (left panel: Ponceau-stained blotting membrane; right panels: immunoblot analyses) demonstrating that two different antibodies raised against AtDOG1 (α-DOG11 and α-DOG12) detect a recombinant 37 kD LesaDOG1* antigen which corresponds to a 37 kD protein purified from an extract of E. coli expressing GST-LesaDOG1 fusion protein ('DOG1'; for constructs and purification procedure see Supplemental Figs. S3 and S4). Note that in the E. coli 'DOG1'-extract the α-DOG1 antibodies detects the strongest 37 kD protein band (Ponceau) as LesaDOG1* (blue arrows), while this antigen is not detected by an α-GST antibody. The '*' indicates the presence of a ca. 3 kD residual linker peptide in the recombinant LesaDOG1* protein left after GST cleavage (Supplemental Fig. S3). An apparent molecular mass of 34 kD is therefore expected for LesaDOG1. Both α-DOG1 antibodies detect 34 kD LepaDOG1 antigens in a L. papillosum protein extract from type-I FO prior to dormancy induction (red arrows). Note that the two α-DOG1 antibodies differ in sensitivity and that the highly similar 34 kD LepaDOG1a and c proteins cannot be distinguished. kD, protein molecular mass marker ladder. | ||
| Article in PDF format (2 MB) Supplementary data file (4.9 MB) |
|
|
|
The Seed Biology Place |
Webdesign Gerhard Leubner 2000 |