New Phytologist 184: 885-897 (2009)
The NADPH-Oxidase AtrbohB plays a role in Arabidopsis seed after-ripening [W]
Centro de Biotecnología y Genómica de Plantas (UPM-INIA), Universidad Politécnica de Madrid, 28223 Pozuelo de Alarcón (Madrid), Spain (M.A.T.)
Received April 30, 2009; accepted July 13, 2009; published online September 15, 2009
DOI 10.1111/j.1469-8137.2009.03005.x
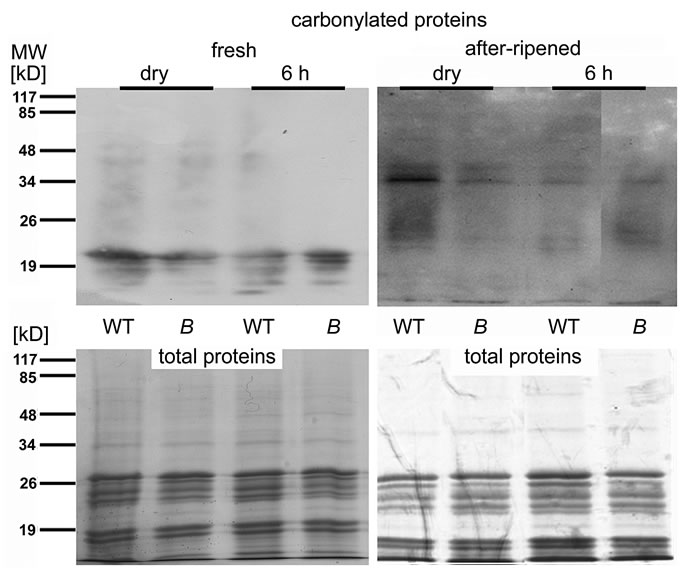
Figure 6. Protein carbonylation is reduced in after-ripened seeds of the atrbohB mutant compared to WT. Immunoblots with antibodies against derivatives of carbonylated (=oxidized) proteins. Protein extracts from dry seeds and seeds after 6 h imbibition of wildtype (WT) and atrbohB (B). The seeds had been grown together and after-ripened in the same location for two years. Below the blot, a Coomassie-stain is shown as a loading control. The majority of the stained proteins most likely correspond to the very abundant globulin storage protein. MW, apparent molecular mass in kD.
| Article in PDF format (440 KB) |
|
|
|
The Seed Biology Place |
Webdesign Gerhard Leubner 2000 |